|
|
Petroleum and Chemical Consulting and Modeling for Enhanced Oil Recovery |
|---|---|---|
Houston, TX - Phone: (281) 564 - 8851 |
||
Polymer Profile Control Technology |
|||
|
Polymers are used in oil reservoirs for three purposes. The first is to increase the viscosity of water and then improve the sweep of water in the reservoir. The second is as a gelled polymer to stop flow of water in and out of sweep zones. The third reason is to improve reservoir sweep in a chemical flood. The discussion of polymer flooding in this section will not address combinations with other chemicals but should be directly applicable in chemical flooding. |
||
Effect of Salinity on Polymers |
Effect of Shear Rate on Polymer Resistance Factor |
||
|
|
||
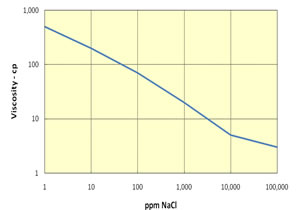
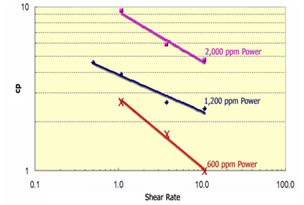
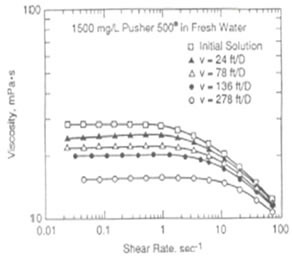
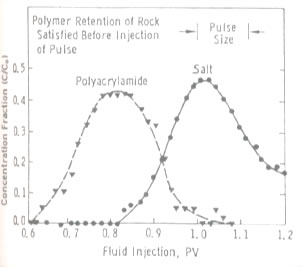
Polymer can either be used alone as a profile modification or blocking agent if gelled, or as part of the chemical slug and chase fluid in an ASP, SP or AP flood. Like chemicals the polymer is affected strongly by how it is used. The figure on the left shows the effect of salinity on the viscosity of 600 ppm of hydrolyzed polyacrylamid (HPAM). This figure shows that sodium chloride has a very large effect on the viscosity and the viscosity decreases an order of magnitude for each factor of 100 increase in salinity. So, if 600 ppm of HPAM where added to fresh water, its salinity would be 500 ppm, while the same concentration in 20,000 ppm could have a viscosity of 3 cp. This is very important because salinity gradients are used to help mobilize oil and especially because alkali chemicals such as sodium carbonate are both adsorbed on the rock and converted to sodium bicarbonate as they propagate. So, the salinity increases while the chemical slug is being injected, then decreases when a polymer chase solution with lower salinity pushes the oil from the rock. Other aspects of polymer behavior are shown in the figure on the right. Polymer solutions are non-Newtonian liquids whose viscosity decreases as the shear rate increases. This is a good thing because it makes it easier to inject the polymer solution. If a polymer injected in low salinity brine were a Newtonian liquid with constant viscosity then the resistance near the injection well would be high and less fluids could be injected. However, as seen below the viscosity of a 2,000 ppm polymer solution decreases by 40 percent as the frontal advance rate increases from 0.5 ft/day (0.152 m/d) to 10 ft/day (3.06 m/d). The velocity of fluids close to an injection well is an order of magnitude higher and the resistance to flow is even lower. The figure above on the right also shows how polymer concentration affects the viscosity. When large polymer molecules are injected into a rock, they might not be able to enter the smaller pores. Thus, an excluded pore volume is sometimes observed. The figure below on the right illustrates that polymer can arrive before the injected brine because of some of the pore volume was inaccessible. In this experiment a polymer solution containing 2 percent NaCl was displaced through a core until no more polymer was retained. Then the salt and polymer concentration were decreased for 0.1 PV. The midpoint of the salt peak in the figure is at the pore volume expected for the plug. However, the change in polymer concentration is reported before the change in salt concentration. This shows that 20 percent of the core was not accessible to the polymer. |
|||
Effect of Shear Degradation on Viscosity |
Effect of Inaccessible Pore Volume |
||
|
|
||
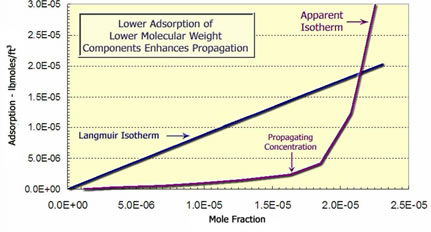
As seen below, polymers are adsorbed on the reservoir rock. Larger polymer molecules are more strongly adsorbed because they cover a larger area. The higher molecular-weight polymer is preferentially adsorbed on surfaces. This desorbs the lower molecular weight polymer and causes it to move through the porous media with less delay. In this case, the chemical concentration which propagates easily is determined by the concentration at which the slope of the adsorption isotherm increases. This happens because the propagation velocity of a chemical is inversely proportional to the slope of the adsorption isotherm. So concentrations with a lower slope propagate faster. |
|||
Langmuir Adsorption Isotherm and Convex Isotherm Calculated from Laboratory Experiment |
|||
|
|||